What do we know about the Russian vaccine
This was published by The Lancet magazine, based on a preliminary study carried out by researchers from the Russian government. This study was evaluated by a rereading committee of the British medical journal prior to its publication.
All reported adverse events on the Russian vaccine were mostly mild. However, a comparative clinical study with other vaccines is needed to confirm these findings, the publication notes.
The information is released after the Russian authorities announced, last August, their vaccine against Covid-19, which they call Sputnik V, in honor of the first satellite launched into space, in 1957.
On August 11, it was reported that Sputnik V was entering the third and final phase of clinical trials.
What elements emerge from the article published in The Lancet? Sputnik V consists of two components: recombinant adenovirus type 26 (rAd26) and recombinant adenovirus type 5 (rAd5); and that both rAd26 and rAd5 contain the SARS-CoV-2 virus protein S gene.
It should be explained that adenoviruses are a group of viruses that typically cause respiratory diseases, such as colds, conjunctivitis (eye infection), bronchiolitis, pneumonia, among others. Both components were developed, manufactured and stored by the National Research Center for Epidemiology and Microbiology NF Gamaleya (Moscow).
During vaccination, component 1 is introduced for the first time and, after three weeks, component 2. According to the evaluation of the developer, the immunity can last up to two years. Then you may need to vaccinate again. However, this remains to be explored in post-marketing studies, explained Vadim Tarasov, director of the Institute of Transmission Medicine and Biotechnology and head of the department of pharmacology at Ivan Sechenov University.
How were the studies done?
It was precisely at the Ivan Sechenov University that preclinical trials of two dosage forms of the Russian vaccine were carried out. The other place where the studies took place was at Burdenko Hospital.
Healthy adult volunteers (men and women), whose ages ranged from 18 to 60 years old, were enrolled for the Russian vaccine studies. Between June 18 and August 3, 2020, they enrolled 76 participants in the two studies Thus, in each study, 38 volunteers were vaccinated. More men than women participated in the study.
According to Tarasov, the study itself was carried out in two stages. One of them was the observation of volunteers for 14 days in isolation to prevent them from contracting the coronavirus infection. Once the observation was completed and it was confirmed that the volunteers did not have Covid-19, they were transported, with all possible precautions, to the cardioangiology center of the Ivan Sechenov University.
In this hospital, 38 volunteers stayed 28 days after the first injection of the drug and were discharged one week after the second. Their stay in the hospital was organized to protect them from the possibility of becoming infected and thus dropping out of the study or entering incorrect data on the immunity developed, he explained.
It was extremely important work, to reliably assess the response of the immune system and draw preliminary conclusions about the effectiveness of the drug, says Sechenov.
According to the study published in The Lancet, the most common reactions among participants were pain at the injection site, hyperthermia (body temperature 37–38 ° C), headache, fatigue, and muscle and joint pain. No serious adverse events were reported during the investigation.
Notably, the volunteers had no infectious diseases at the time of vaccination and for 14 days prior to vaccination, and did not receive any other vaccines within 30 days of participating in the study.
Caution
When the world announcement of Sputnik V was made, it was greeted with skepticism by many researchers by the scientific community. Its efficacy and safety were questioned, mainly due to the absence of public data on the trials conducted up to that point.
This opinion is largely due to misunderstanding that the preparation in question is registered in the Russian Federation 'conditionally', says Sechenov. As he explains, this is a well-known and widely used mechanism in the United States, the European Union, Japan and other countries with a developed regulatory system. Its essence lies in the fact that if it is necessary to put the drug into practice as soon as possible, for example, for orphan or oncological diseases, during epidemics, etc., and it is impossible to conduct full clinical trials, the drug is temporarily registered with indications of limited use, which are based on the results of clinical trials already carried out, he details.
At the same time, a mandatory requirement is to conduct post-registration studies with the participation of a certain number of patients at a given time, and any use of the drug becomes part of the study.
This is the case of the Sputnik V vaccine, whose registration certificate was issued until January 2021, and as a prerequisite, clinical trials with 40,000 participants were established, says Sechenov.
As has been announced, this will be the objective of the phase 3 trial (which will include 40,000 participants), of various ages and with different risk levels.
Since the announcement made by President Vladimir Putin last August, the World Health Organization (WHO) has urged Russia to follow the established protocol and comply with all the phases necessary to develop a safe vaccine.
The WHO will not endorse a vaccine against the coronavirus if it is not safe and effective, declared this Friday, September 4, its director general, Tedros Adhanom Ghebreyesus.
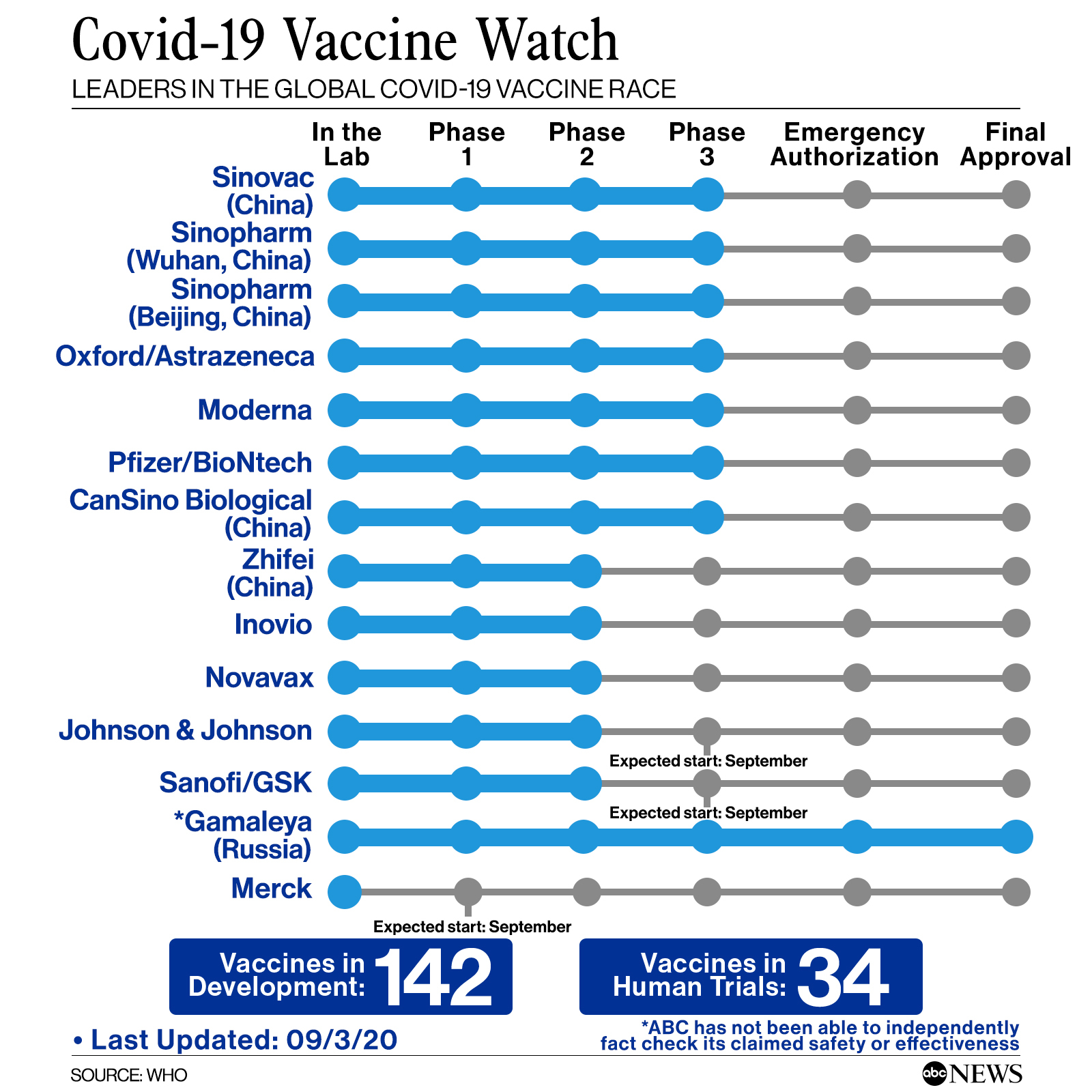
According to the WHO, there are 176 vaccine projects underway in the world, of which 34 are in the clinical trial phase, which means that they have started to be tested in humans. Among these, eight are in phase three, the most advanced.
Around the world, the pandemic has claimed so far the lives of more than 875,700 people and infected more than 26.6 million.