
UK experts back AstraZeneca jab amid Germany ruling
Dr Mary Ramsay of PHE said the jab offers "high levels of protection" against Covid-19, particularly against severe illness.
Boris Johnson said he was not concerned by Germany's recommendation.
AstraZeneca said the jab's trial data supported efficacy in the over-65s.
Germany's vaccine committee said the AstraZeneca vaccine should only be given to people aged under 65, citing a lack of sufficient data to recommend use among older age groups.
The European Medicines Agency (EMA) is to decide on Friday whether to approve the vaccine for use across the EU.
But Dr Ramsay, head of immunisations at Public Health England, said: "Both the AstraZeneca and Pfizer-BioNTech vaccines are safe and provide high levels of protection against Covid-19, particularly against severe disease.
"There were too few cases in older people in the AstraZeneca trials to observe precise levels of protection in this group, but data on immune responses were very reassuring."
Mr Johnson said he was not concerned by Germany's recommendation, adding that the UK's watchdog, the Medicines and Healthcare products Regulatory Agency (MHRA), had "made it very clear" that the AstraZeneca vaccine is "very good and efficacious" and gives a "high degree of protection after just one dose, and even more after two doses".
Speaking during a visit to Scotland, the prime minister added: "The evidence that they've supplied is that they think it is effective across all age groups [and] provides a good immune response across all age groups, so I don't agree with that [Germany's recommendation]."
An AstraZeneca spokesperson said the latest analyses of clinical trial data for its vaccine "support efficacy in the over 65 years age group", adding that the firm was awaiting "a regulatory decision on the vaccine by the EMA in the coming days".

All of the regulators and experts in different countries have been looking at the same data on the Oxford-AstraZeneca vaccine.
That data comes from clinical trials, and those did recruit fewer elderly people overall.
That's because they started off first with younger volunteers to get results as quickly as possible, given the urgency to find out if a vaccine would work well enough to help get us out of the pandemic.
The scientists who ran the trials have always been upfront about this.
But they say there is other evidence to suggest the vaccine will work well in older adults.
Studies show the over-65s have a strong immune responses to the vaccine. After receiving the shots their blood has plenty of the required antibodies that can fight coronavirus.
The UK has been using the AstraZeneca vaccine in its mass immunisation programme for weeks now and should soon have more proof from the real world setting about how much protection the shots give.
Prof Paul Hunter, of the University of East Anglia, told BBC News that the elderly should not worry about receiving the jab.
He said: "I'm almost 65 myself and I would happily take any of the vaccines, including the Oxford-AstraZeneca vaccine. We do know that it is safe in people over 65.
"They have much fewer side effects than younger people and it almost certainly provides substantial benefits in terms of preventing severe disease and reducing the chances of going into hospital."
'No concerns' over safety
Prof Adam Finn, a member of the Joint Committee on Vaccination and Immunisation (JCVI), told BBC's Radio 4's PM programme that Germany's recommendation "just reflects different levels of caution", adding: "What they are basically doing is saying 'We'd like to wait a big longer and know a bit more before we move."
Prof Finn, professor of paediatrics at the University of Bristol, said: "We have no concerns about the safety of the vaccine in any age group".
Dr Doug Brown, chief executive of the British Society for Immunology, said the MHRA would have "carefully scrutinised the evidence" on the AstraZeneca jab before making their recommendation, and referred to its original report which stated that there is "there is limited information available on efficacy in participants aged 65 or over, although there is nothing to suggest lack of protection".
He said this reflected a "need for more data" on the effectiveness of the jab in this age group, but noted the jab had shown a "good safety profile" in all published reports.
Prof Jim Naismith, director of the Rosalind Franklin Institute at the University of Oxford, said German scientists had "not said the vaccine is ineffective for over-65s".
He added: "Scientists often disagree about how much evidence is needed for any new advance and there is always more data to be secured.
"Normally this all happens out of sight of the glare of the media and not in a pandemic, but such debates are an important part of the scientific process that is familiar to anyone who has ever been through peer review."
Meanwhile, the EU and AstraZeneca are involved in a row over vaccine supply shortages.
AstraZeneca has previously said it could deliver only a fraction of the doses between January and March that it had promised to the bloc, blaming production issues at EU plants for a reported 50 million-dose shortfall.
The EU has demanded that UK-made jabs are diverted to mainland Europe to fulfil contractual obligations.
However, both sides pledged to work together to resolve the crisis.
But Cabinet Office minister Michael Gove has said there would "be no interruption" to UK vaccine supplies.
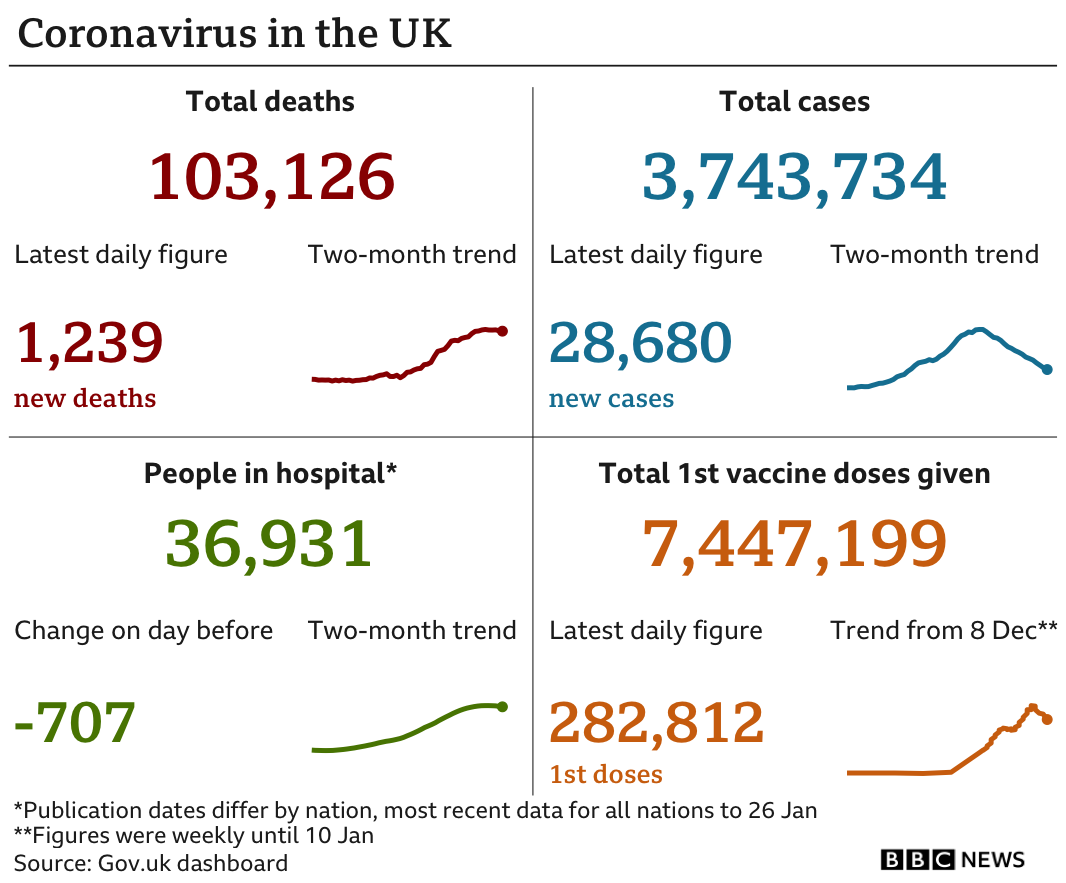
It comes as the UK recorded a further 1,239 deaths within 28 days of a positive coronavirus test on Thursday, according to government figures. There have also been another 28,680 new infections.











