
There Are New Safety Concerns About Russia's COVID Vaccine, Which Is Already Being Distributed Worldwide
The global drive to speed COVID-19 vaccination has taken a serious blow since Brazilian health officials on Monday recommended against importing Russia’s Sputnik V vaccine to curb a severe coronavirus epidemic. In an extraordinary dispute that blew up on Thursday, the vaccine’s Russian backers threatened legal action for defamation and the Brazilian officials released recordings and documents supporting their position.
As the coronavirus pandemic continues to rage across the world, the angry exchanges have raised questions about the safety of the shots and doubts about its maker’s willingness to answer them. That poses a big headache for dozens of countries that have already accepted donations of the vaccine from Russia in hopes of accelerating their vaccination programs.
Officials with Anvisa, Brazil’s health regulatory agency, said Monday that documentation provided by the Gamaleya Research Institute in Moscow didn’t provide enough information about the safety and efficacy of the Sputnik V vaccine — including information on any serious side effects. The documents also indicated that the inactivated cold viruses the vaccine relies on to deliver an immune response against the coronavirus, which are supposed to be unable to reproduce, were in fact able to do so.
This finding shocked vaccine experts. If confirmed, it suggests manufacturing was botched and that the Sputnik V vaccine is unlikely to win approval from other leading regulators around the world.
“If this is true, Sputnik is nixed,” John Moore, a virologist at Weill Cornell Medical College in New York, told BuzzFeed News.
Russian authorities reacted angrily to Anvisa’s ruling. “The decision by Brazil’s National Health Surveillance Agency (Anvisa) to delay the approval of Sputnik V is, unfortunately, of a political nature and has nothing to do with the regulator’s access to information or science,” said a statement posted on the website set up to promote the vaccine.
By Thursday, things had escalated into a bitter public dispute. To counter Russian claims that it was spreading “fake news” about Sputnik V, Anvisa took the highly unusual step of releasing a recording of parts of a teleconference with Gamaleya officials. The vaccine’s official Twitter account had previously gone on the offensive, controversially claiming other vaccines have a poorer safety record, sparring with a leading virologist who commented on Anvisa’s concerns, and stating that the vaccine’s makers would launch a defamation action against the Brazilian agency for “knowingly spreading false and inaccurate information.”
In a presentation posted on the Brazilian government’s YouTube channel on Thursday, Anvisa officials showed portions of the Russian documents they reviewed that mentioned the presence of viruses that were able to reproduce. The video also showed part of a three-hour April 23 teleconference in which Anvisa officials said they asked for more information but did not receive satisfactory answers.
For the process of vaccine approval to degenerate into a public spat with threats of legal action is highly unusual. “I don’t think I’ve ever seen anything like this,” Monica de Bolle, an economist from Brazil working at the Peterson Institute for International Economics in Washington, DC, told BuzzFeed News.
“If they want to sue us, then sue us,” Antonio Barra Torres, head of Anvisa, told reporters on Thursday. “We’ll answer through the correct channels.”
Sputnik V has been controversial from the start. It was approved for use in Russia last August before clinical trials were completed. That gamble seemed to have paid off in February, however, when a paper published in the Lancet, a medical journal, indicated the vaccine was 91.6% effective in preventing people from getting sick with COVID-19.
The Sputnik V vaccine consists of two doses of common cold viruses called adenoviruses; the first dose is a virus called Ad26 and the second Ad5. In the vaccine, they are modified so they make the “spike” protein from the coronavirus, priming the immune system to attack it. The vaccine’s adenoviruses are also supposed to lack two key genes that they need to reproduce.
This is the same basic technology behind the vaccines made by Johnson & Johnson, which uses a single dose of a modified Ad26 virus, and AstraZeneca, which uses two doses of a different adenovirus that normally infects chimpanzees.
Buoyed by the results of the clinical trial, the Russian Direct Investment Fund (RDIF), set up by the Kremlin to invest in homegrown companies, has offered the vaccine to dozens of countries across the world in a major diplomatic push, especially in the developing world. The Sputnik V Twitter account boasts that the vaccine has been authorized in more than 60 countries.
But many of those countries lack strong expertise for judging the safety and efficacy of new drugs and vaccines, and they tend to follow the leads of the World Health Organization, the FDA, or the European Medicines Agency. None of these organizations have yet given a green light to use Sputnik V.
The EMA announced a “rolling review” of Sputnik V on April 3. But on Monday, German Chancellor Angela Merkel said that insufficient information had yet been provided for the European Union to authorize the vaccine. Nevertheless, two EU members, Hungary and Slovakia, have started using Sputnik V. But Slovakia rejected a batch of the vaccine earlier this month after its “characteristics and properties” were found to be different from the shots described in the Lancet paper.
No date has been set yet for the WHO’s review of the vaccine. “On Sputnik, we are still waiting, we are still in the back-and-forth stage,” WHO spokesperson Margaret Harris told a briefing in Geneva on Tuesday, Reuters reported.
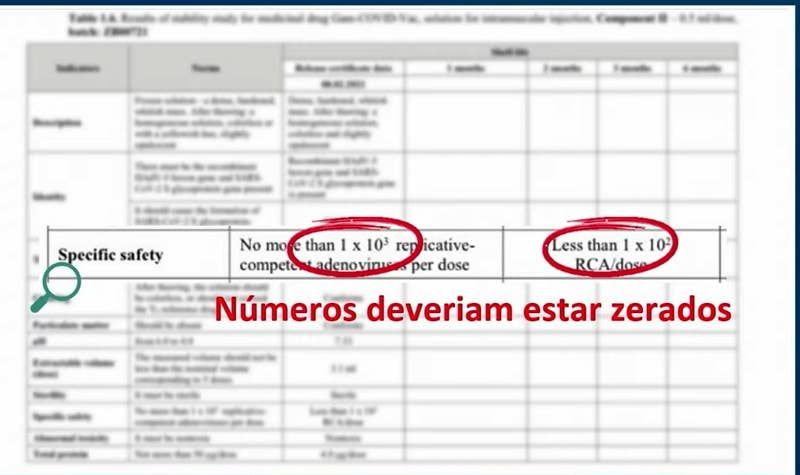
So the review by Anvisa was Sputnik V’s first big regulatory test. In Thursday’s YouTube video, Gustavo Mendes, Anvisa’s general manager for medicines and biological products, showed documents supplied by the Gamaleya Research Institute. These noted that the vaccine could contain up to 1,000 viruses able to reproduce per dose and that tested samples contained fewer than 100. “Those numbers should be zero,” he says.
Even before this week’s dispute, Russia’s donations of the vaccine to developing nations before the world’s leading regulatory authorities approved it had alarmed some experts. One concern is that many of these countries lack good systems to look for adverse events such as the rare but very serious blood clots triggered by the similar vaccines made by AstraZeneca and Johnson & Johnson.
“This creates a lot of concern for us in the global vaccine community,” Peter Hotez of the Baylor College of Medicine in Houston, who has been heavily involved in developing vaccines for countries without strong healthcare infrastructure, told BuzzFeed News.
Even if the cold viruses used in the Sputnik V vaccine are able to reproduce, it is unlikely to cause serious illness unless the recipients are badly immunocompromised, like some patients with HIV or organ transplants. But failure to completely inactivate the viruses would be a red flag against the manufacturing of the Sputnik V vaccine.
“It’s a big nyet-nyet,” Moore said.
Despite the pushback from Russia, Anvisa’s experts are respected internationally for being thorough. And for Brazil’s regulator to raise concerns as the nation wrestles with a COVID-19 outbreak that is currently killing around 2,500 Brazilians a day, driven by a highly contagious coronavirus variant, is significant. The official death toll in Brazil passed 400,000 this week.
“There is not, in this institution, any person that has any interest or joy in denying the import of any vaccine,” Torres says in the YouTube video.
Possible viral replication isn’t the only concern about Sputnik V. In October, four senior AIDS researchers noted in a letter to the Lancet that a decade ago they had abandoned a clinical trial of an HIV vaccine using an inactivated Ad5 virus for safety reasons: In men who had previously been infected with Ad5, the vaccine actually increased susceptibility to infection with HIV.
“On the basis of these findings, we are concerned that use of an Ad5 vector for immunisation against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) could similarly increase the risk of HIV-1 acquisition among men who receive the vaccine,” the scientists wrote.
One of those researchers, Carl Dieffenbach, director of the division of AIDS at the National Institute of Allergy and Infectious Diseases in Bethesda, Maryland, told BuzzFeed News that he would avoid using an Ad5-based vaccine in countries like those in Africa and Latin America where HIV infection is a serious concern.
“I think it’s unnecessary,” Dieffenbach said. “Ad26 is a perfectly fine vector in itself. It doesn’t do this.”
In addition to Sputnik V, Ad5 is used as the vector for a COVID vaccine made by the Chinese company CanSino, which has been authorized for use in countries including Pakistan, Mexico, and Chile.
Despite the emerging concerns about the safety of Sputnik V, the vaccine is still in demand in many countries battling COVID-19. The RDIF announced that it will start shipping the vaccine to India, which is now in the grip of a devastating coronavirus outbreak, on May 1. Turkey announced on Friday that it had authorized the vaccine for emergency use.
The RDIF did not immediately respond to requests for further comment on the safety concerns or the threats of legal action against Anvisa.
“I think we’ve expressed our position in tweets and other statements,” Gleb Bryanski, the RDIF’s director for special projects, told BuzzFeed News.
Comments











